High Profile Diseases - Induced Pluripotent Stem Cells for Regenerative Medicine
Jordana Lenon (WNPRC)
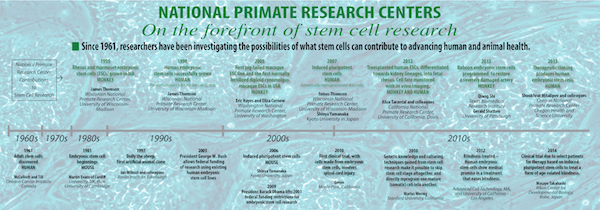
The National Primate Research Centers have been responsible for groundbreaking advances in pluripotent stem cell research since 1995. (See timeline.) Scientists use pluripotent stem cells as a basic research tool to understand the human body, for pharmaceutical development and drug toxicity testing, to screen for environmental compounds that may be harmful to humans, and for preclinical tissue and organ transplant studies. Embryonic stem cells come from leftover lab-fertilized embryos donated by patients at in vitro fertilization clinics. Induced pluripotent stem cells (iPS cells) are genetically reprogrammed skin, fat or other mature cells that act like embryonic stem cells but can be derived from an individual’s own cells to study and potentially treat a particular genetic or degenerative disease in that individual.
As pluripotent stem cell research moves from bench to bedside, large animals such as nonhuman primates, pigs and sheep are becoming more important for preclinical studies. For example, researchers are using heart, bone, and retinal cells grown from both ES and iPS cells to regenerate healthy cardiac tissue after myocardial infarction, lost bone and cartilage due to joint injury or degeneration, and retinal cells to restore vision in patients with juvenile and age-related macular degeneration.
Pluripotent stem cells can also be used to grow mesenchymal stem cells, which are normally found in bone marrow but are difficult to extract in large quantities for basic and preclinical research. Clinicians hope to grow large numbers of mesenchymal stem cells from a patient’s own iPS cells, to regenerate bone marrow for cancer treatment: There are not enough matched bone marrow donors and even autologous (self) bone marrow extraction, cryopreservation and transplant after cancer treatment is a risky process and not always successful. Mesenchymal stem cells and related types of stem cells are showing potential for therapeutic, protective and regenerative benefits in other tissues and organs as well.
NPRC scientists are also using iPS cells to grow dopaminergic neurons for researching Parkinson’s disease therapies, pancreatic beta cells for diabetes studies, and motor neurons to study ALS. One NPRC scientist is interested in using iPS cells as a source of cells to produce oocytes for fertility treatment in cancer survivors who suffer from ovarian insufficiency. Nonhuman primate models will be the critical stepping stone between many of these basic stem cell research studies and human treatments.
Click image to enlarge.
Article References
Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Becker RA, Hearn JP. Isolation of a primate embryonic stem cell line. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):7844-8.PMID: 7544005. PMC41242.
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998 Nov 6;282(5391):1145-7. Erratum in: Science 1998 Dec 4;282(5395):1827. PMID: 9804556. PMCID unavailable.
Zhang SC, Wernig M, Duncan ID, Brüstle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001 Dec;19(12):1129-33. PMID: 11731781. PMCID unavailable.
Zwaka TP, Thomson JA. Homologous recombination in human embryonic stem cells. Nat Biotechnol. 2003 Mar;21(3):319-21. PMID: 12577066. PMCID unavailable.
Gerami-Naini B1, Dovzhenko OV, Durning M, Wegner FH, Thomson JA, Golos TG. Trophoblast differentiation in embryoid bodies derived from human embryonic stem cells. Endocrinology. 2004 Apr;145(4):1517-24. PMID:14684604. PMCID unavailable.
Vodyanik MA, Bork JA, Thomson JA, Slukvin II. Human embryonic stem cell-derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 2005 Jan 15;105(2):617-26. PMID:15374881. PMCID unavailable.
Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Mitchen ER, Frane JL, Crandall LJ, Daigh CA, Conard KR, Piekarczyk MS, Llanas RA, Thomson JA. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006 Feb;24(2):185-7. PMID:16388305. PMC unavailable.
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007 Dec 21;318(5858):1917-20. PMID: 18029452. PMCID unavailable.
Choi KD, Yu J, Smuga-Otto K, Salvagiotto G, Rehrauer W, Vodyanik M, Thomson J, Slukvin I . Hematopoietic and Endothelial Differentiation of Human Induced Pluripotent Stem Cells. Stem Cells. 2009 Mar 2;27(3):559-567. PMID: 19259936. PMC2931800.
Tarantal AF, Lee CC, Batchelder CA, Christensen JE, Prater D, Cherry SR. Radiolabeling and in vivo imaging of transplanted renal lineages differentiated from human embryonic stem cells in fetal rhesus monkeys. Mol Imaging Biol. 2012 Apr;14(2):197-204. PMID: 21479709. PMC4224287.
Shi Q, Hodara V, Simerly CR, Schatten GP, VandeBerg JL. Ex vivo reconstitution of arterial endothelium by embryonic stem cell-derived endothelial progenitorcells in baboons. Stem Cells Dev. 2013 Feb 15;22(4):631-42. PMID: 22931470. PMC3564485.
Emborg ME, Liu Y, Xi J, Zhang X, Yin Y, Lu J, Joers V, Swanson C, Holden JE, Zhang SC. Induced pluripotent stem cell-derived neural cells survive and mature in the nonhuman primate brain. Cell Rep. 2013 Mar 28;3(3):646-50. PMID: 23499447. PMC3633566
Tachibana M, Amato P, Sparman M, Gutierrez NM, Tippner-Hedges R, Ma H, Kang E, Fulati A, Lee HS, Sritanaudomchai H, Masterson K, Larson J, Eaton D, Sadler-Fredd K, Battaglia D, Lee D, Wu D, Jensen J, Patton P, Gokhale S, Stouffer RL, Wolf D, Mitalipov S. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell. 2013 Jun 6;153(6):1228-38. PMID: 23683578. PMC3772789.
Fox IJ, Daley GQ, Goldman SA, Huard J, Kamp TJ, Trucco M. Stem cell therapy. Use of differentiated pluripotent stem cells as replacement therapy for treating disease. Science. 2014 Aug 22;345(6199):1247391. Review. PMID25146295. PMC4329726.
Salih SM, Ringelstetter AK, Elsarrag MZ, Abbott DH, Roti EC. Dexrazoxane abrogates acute doxorubicin toxicity in marmoset ovary. Biol Reprod. 2015 Mar;92(3):73. PMID: 25609833. PMC4367967.
Pellett S, Schwartz MP, Tepp WH, Josephson R, Scherf JM, Pier CL, Thomson JA, Murphy WL, Johnson EA. Human Induced Pluripotent Stem Cell Derived Neuronal CellsCultured on Chemically-Defined Hydrogels for Sensitive In Vitro Detection of Botulinum Neurotoxin. Sci Rep. 2015 Sep 28;5:14566. PMID: 26411797. PMC4585966.